Across the kingdom of life, an increase in intracellular levels of NAD+ triggers shifts that enhance survival, including boosting energy production and upregulating cellular repair. By middle age, our NAD+ levels have plummeted to half that of our youth. Numerous studies have demonstrated that boosting NAD+ levels increases insulin sensitivity, reverses mitochondrial dysfunction, and extends lifespan.
In most eukaryotic systems, NAD+ can be synthetized de novo from the amino acid tryptophan. Otherwise, it can be produced via salvage pathways requiring a preformed pyridine ring. Three naturally occurring vitamins of the vitamin B3 group (“niacin”) can serve as dietary precursor molecules for NAD synthesis:
-
nicotinic acid (NA or niacin),
-
nicotinamide (NAM or niacinamide),
-
nicotinamide riboside (NR)
This vitamine B derived NAD synthesis occurs via the Preiss–Handler pathway (NA) and the salvage pathway (NAM and NR).
NA riboside (NaR) is another, yet largely unstudied NAD+ precursor.
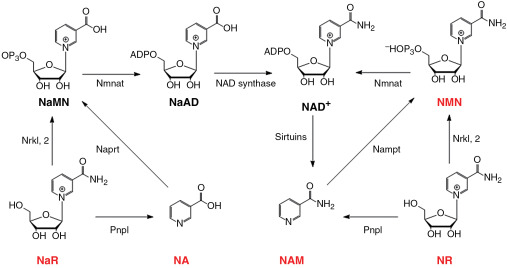
Synthesis of NAD+ from both NAM and NR is a two-step process, where during the first step both of these molecules are transformed into nicotinamide mononucleotide (NMN). The reactions leading to NMN production are however different: while NAM is converted into NMN by NAM phosphoribosyltransferase (NAMPRT), NR is phosphorylated by nicotinamide riboside kinase (NRK). Two isoforms of NRK exist in mammals: NRK1, which is ubiquitously expressed and NRK2, which is mainly expressed in liver, skeletal muscle, heart, and BAT. NMN is then transformed into NAD+ by NMN adenylyltransferase (NMNAT). Three different isoforms of this enzyme exist and show differential tissue expression: NMNAT1 being ubiquitously expressed, with the highest activity detected in liver and kidney, NMNAT2 expression limited to the brain and NMNAT3 presence detected in multiple tissues, similarly to NMNAT1, but to a much lesser extent.
NA is converted into nicotinic acid mononucleotide (NaMN) by the nicotinic acid phosphoribosyltransferase enzyme (NAPRT). NaMN is subsequently converted into nicotinic acid adenine dinucleotide (NaAD), also called desamido NAD+, by nicotinamide mononucleotide adenylyl transferase enzymes (NMNAT). Finally, NaAD is converted into NAD+ by the NAD+ synthetase enzyme (NADS).
Based on the tissue distribution of the enzymes involved in the de novo NAD+ production, it is generally assumed that NAD+ synthesis from tryptophan is limited to liver, kidney, and possibly the brain.
Boosting NAD+ levels is largely beneficial and modulates cellular senescence and ageing. Thus, modulation of NAD+ production is anticipated to prolong both health span and life span.
Pathways of NAD+ biosynthesis in mammalian cells. NAD+ precursors (red) can be used to enhance NAD+ concentrations.
NA: Nicotinic acid
NaAD: nicotinic acid adenine dinucleotide
NAM: nicotinamide
NaMN: nicotinic acid mononucleotide
Nampt: nicotinamide phosphoribosyltransferase
NMN: nicotinamide mononucleotide
NR: nicotinamide riboside
Which of these NAD+ precursors (boosters) are the most successful as a longevity agent?
For proof of concept pharmacology studies, the starting points and frames of reference are provided by the free base precursors NA and NAM. Importantly, NA has been widely used (in humans) for treatment of hyperlipidemias and cardiovascular diseases, although it can cause uncomfortable flushing with even intermediate doses. Nevertheless, recent studies indicate that NA can increase NAD+ concentrations in cell culture and in mice tissues. Moreover, a new study reports NA improves genome stability. NAM is the most common precursor form of NAD+ in mammalian cells and tissues. NAM exhibits variable effects on NAD+ concentrations in different tissues. Liver rapidly converts NAM into increased NAD+, whereas in other tissues NAM has limited NAD+ enhancing effects. NAM also inhibits sirtuins, making it seemingly less useful as an NAD+ precursor for enhancing sirtuin activity. While some of the data for NA and NAM are quite positive, both NR and NMN appear to exert more potent effects on NAD+ synthesis, suggesting greater potential for these newer NAD+ precursors in longevity applications.
NR can strongly promote the biosynthesis of NAD+ in mammalian cells and tissues. By this means, NR increases the catalytic activity of NAD+-dependent sirtuin activities. It has been demonstrated that NR is a potent stimulator of NAD+ production in various mammalian cell lines from 170% to 270% of controls. NR prevents high fat diet-induced obesity and protects mice from noise-induced hearing loss, DNA damage, and can improve lifespan in an animal model of heart failure. NR treatment in a mouse model of Alzheimer disease, significantly increased the NAD+ level in the cerebral cortex and improved cognitive function. NR supplementation in old mice (24 months of age) resulted in a significant increase (∼40%) in additional lifespan (as measured at the time treatment started). Importantly, a clinical study was published recently found NR is safe at dosages of up to 1000 mg/kg (in humans) taken for months. These findings suggest that NR can serve as a potent agent for the treatment of aging and aging-related metabolic disorders.
NaR, synthesized from nicotinic acid riboside ethyl ester, has also been identified as a NAD+ precursor in yeast and mammalian cells, through both Nrk-dependent and Nrk-independent pathway to produce NAD+. It has been reported that NaR treatment increases intracellular NAD+ concentrations in three different mammalian cell lines by 150%–190% of controls, although in yeast NAD+ precursor activity of NaR depends upon degradation. NaR has the potential to be important mammalian nutritional precursor, and could enhance NAD+-dependent benefits provided by sirtuins, although its effects still need to be determined.
NMN can also serve as a potent NAD+ precursor, particularly because it has been observed to attenuate age-associated physiological problems and other disease conditions in mice. The effects of NMN as an NAD+ raising agent are looking very promising in varies studies. This suggests that NMN could be an effective precursor of NAD+ in vivo. It has been shown that glucose-stimulated insulin secretion can be corrected by administration of NMN in genetic mouse models or aged mice. Treatment with NMN protects old mice against diet- and age-induced diabetes and restores skeletal muscle mitochondrial function to youthful levels. NMN also protects the heart from ischemia and reperfusion injury, and ameliorates cognitive impairment and neuronal death in Alzheimer's disease model rats.
Scientists continue to debate which of the two biosynthetic precursors, NMN or NR, is superior regarding safety and efficacy. Due to the size of NMN, some scientists believe it must convert to NR before crossing the cell membrane to enter cells. But scientists from Washington University School of Medicine recently provided compelling evidence for the existence of a specific transporter for NMN in the gut of mice called Slc12a8. A transporter is a protein which allows the smooth passage of a molecule across a cellular barrier, such as a cell membrane.
Currently, scientific evidence seems to suggest that NMN is probably slightly better than NR. This makes sense, because NMN is further down the NAD+ production pathway. Molecularly speaking, NMN looks more like NAD+ than NR does. Additionally, studies show impressive results of NMN on many aging mechanisms, more so than NR.
According to studies performed thus far, no safety concerns exist either for NMN or NR consumption.
Literatuur:
- Sirtuins in Health and Disease; Ning Zhang, Anthony A. Sauve, in Progress in Molecular Biology and Translational Science, 2018
- https://www.sciencedirect.com/topics/medicine-and-dentistry/nicotinamide-riboside
- https://www.nmn.com/precursors/nmn-vs-nr